EOL©, an e-CRF solution for clinical studies and randomization
EOL© a solution adapted to your profile





Choose your profil
Client's testimonial 2014

AFCP - French Foot Association Surgery
"We needed a reliable solution, with an exellent quality-price ratio, reachable from allover the French territory. Medsharing fully satisfied us by its reactivity, its adaptability and its software ergonomy, usable with a very light training.
EOL software is so simple to use, that we even use it directly in the operating room".
Dr Jean-Luc Besse, M.D., Ph.D - Surgeon at Hospices Civils de Lyon and Past-Président, AFCP, member of the EFAS (European Foot and Ankle Society) Executive Committee (2014).
Medical Device

STENTYS successfully collects real-world data for post marketing surveillance in eight European countries
“Medsharing EOL software is easy to work with; for example we can easily extract data and create reports ourselves. Medsharing have always been very responsive to our requests and the eCRF has excellent response times. The screen layout and the inclusion of graphics in the CRF pages facilitate patient data entry for site personnel.”
Click to read the success story ...
EOL on ipad
EOL software is 100% compatible with all new mobile devices such as ipad and iphone but also other smartphones. Our light and compatible with all browser technology makes our solution very easy to use with tablets and smartphones.
Data entry and randomization are directly available on your mobile device anywhere or via your internet connexion.
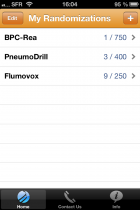
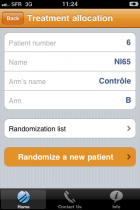
iPhone-iPad randomization
RANDOMIZER for CLINICAL TRIAL, which includes the possibility of exporting directly to Excel your patients lists.
You can download this apps via the appstore keywords : medsharing, randomizer, ...) or you can visit our iPhone/iPad randomization page.
This application, is simple, intuitive and practical, can be used with every type of studies. It allows to create as many study as you want and include an unlimited number of patients.
DIA Annual Meeting Chicago: Feedback
 Medsharing spoke at the Drug Information Association (DIA) Annual Conference on June 19th in Chicago, USA.
Medsharing spoke at the Drug Information Association (DIA) Annual Conference on June 19th in Chicago, USA.
We presented the BIG-RENAPE Oncology Hybrid Study: Benefits of Combining Prospective Data and Existing Retrospective Data in One Place. There were many questions from the audience, from big pharmas like GSK to small biotechs.
To see full details on session agenda and speakers, click here.
DIA is a not for profit association aiming at providing information and training in the life sciences domain. The DIA Annual Meeting is one of the main event in the clinical trials, safety and regulatory arena. It hosts every year 7500 participants from 50 countries, 450 exhibiting companies, 10+ tracks, and more than 160 sessions.
Patients' Surveys: Recruit More with Less Budget!
As you are probably already aware, in order for MAHs to obtain Post Market Registration Renewal, Health Authorities and Regulatory Bodies ask MAHs to provide evidence of product's Benefit-Risk assessment on a regular basis.
In order to do this, sponsors are encouraged to conduct observational studies with real world data. These studies include a patients' survey most of the time.
Most eCRF softwares are rather CDMS (Clinical Data Management System) than pure internet age eCRFs. They were designed more for Data Managers than for clincal sites end users.
Hence they are too costly for observational studies and with a poor user experience, especially on tablets and smartphones.
Medsharing's answer is to offer as a standard in EOL©:
- Same software for eCRF and ePRO ► Time and money saving
- Enhanced user experience with Interactive Images and Contextual Pop-Up Windows ► very short end user trainings and better adherence from site personel and patients
- Illimited Number of End Users included in license price
- On the Fly eCRF Amendments (e.g. adding a page)
- Interpanel Reports
Since 2000 we completed over 80 observational studies in Europe and beyond.
We would be delighted to exchange with you, provide more information, or show EOL© via a webconference ►Please contact Bertrand Le Bourgeois at blb@medsharing.fr
You can also downlaod our latest brochure from our website: www.medsharing.fr
ACDM Annual Conference Brussels

Medsharing spoke and exhibited at the Association for Clinical Data Management (ACDM) annual conference on March 14th in Brussels.
The European eCRF provider presented how its client Stentys, a mid-size medical device company streamlined his non-interventional study in 10 countries in Europe and Asia.
To download the presentation, please click here.
For more information on the conference, please visit acdm.org.uk
(Conference in English)
6th Clinical Research Day - Paris

Medsharing moderated the workshop ‘eHealth and regulations’ and exhibiedt at 6th Clinical Research Day in Paris, France on January, 26th 2017.
29 clinical research professionals attended the eHealth workshop, and then we presented the summary in plenary session in front of 300+ persons!
Thanks to our clients, prospects and partners who attended the conference and visited our stand during the day.
To learn more about the conference proceedings, please contact us.
3sites Study from French Caen University Hospital
The 3sites study, sponsored by French Caen University Hospital, and for which Medsharing’s EOL software has been used, has been published in the New England Journal of Medicine dated 24 September 2015
Intravascular Complications of Central Venous Catheterization by Insertion Site http://www.nejm.org/doi/full/10.1056/NEJMoa1500964
Medsharing at Data Management Biomedical

Medsharing, the eCRF leader, to exhibit at Data Management Biomedical Conference, Paris, Nov 10th 2015.
Topics are Central Monitoring, Clinical trials outsourcing and connected devices.
To know more about Medsharing’s solutions for clinical trials, registries or patients' quality of life questionnaires, please contact us info@medsharing.fr or visit http://www.ecrf-medsharing.com/
Medsharing Signs New US Research Center

Medsharing SARL, the provider of SaaS eCRF and randomisation software EOL© for interventional and observational medical research, announces the signature of a contract with the Osteoporosis Medial Center (OMC) in Beverly Hills, California, USA (www.omcresearch.org).
OMC will be using EOL© for a multicentric Drug Holiday Study in the US.
« We chose Medsharing for its reactivity and support level. Medsharing has provided excellent support and rapid turnaround in the development phase of our eCRF so far. »
– Dr Stuart L. Silverman, MD FACP FACR, Clinical Professor of Medicine, UCLA School of Medicine and Medical Director, OMC Clinical Research Center.
To know more about Medsharing’s solutions, please contact us at info@medsharing.fr
The 100th one!

On the 2nd of April, we launch our 100th one study.
Thank to the Clinical Investigation Center of Grenoble!
eCRF improvment
Do you need informations about eCRF improvment? Go to Designing your study
Don't hesitate to send your feedback on this first randomizer application. A non restricted version will be available soon.
Our news
EOL New brochure available!
March 2016 : EOL the new brochure is available!
Medsharing in figures
(Figures 2015)
78,000 patients since 2003 for over 130 studies.
26 Registries / Observatories.
49 Studies.
61 Clinical Trials
31 Randomizations (without eCRF)
47 Drug Studies
28 DM Studies
66 Ongoing studies.
Figures 2015
New version of the application of randomization
Check out the new unlimited version of our Randomization for clinical trial application IPHONE / IPAD.
Quote request
+33 (0)1 48 75 39 14
by e-mail