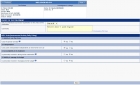
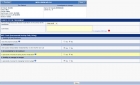
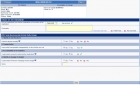
Designing your study
The personalisation of the eCRF is provided entirely by the settings and does not involve specific developments. This ensures the success of your project (no risk of uncontrolled running, no specific software test).
From your paper CRF MEDSHARING uses EOL Conception© to:
- Create the items;
- Associate controls on items (limits, possible values, format ...) ;
- Associate controls between different items (coherence, completeness ...) ;
- Create assistance and information fields (info bubble, comments);
- Associate files in electronic format (doc, pdf, xls….) ;
- Create libraries of standard variables and paragraphs;
- To set real-time alerts via email and SMS (EIG, deviance ...) ;
- Make items conditionals according to the context;
- To set the schedule of visits;
- To set the criteria for inclusion;
- To set randomisation.
Nevertheless, our experience has shown the large diversity of needs in the clinical studies area. EOL Conception© therefore generates contents which we have the flexibility to integrate into specific developments of all kinds
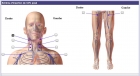
- Interactive pictures
- Visual Analogic Scales
- Comparison of data including validation by an expert
- Masked field
- Calculations (scores, duration, age, BMI)
- eCRF designed regarding our graphic standards
- Possibility to integrate your logo or the logo of the study in the eCRF home page and to add centres specific documentation (local language, newsletters, ...) to increase interactivity
An area dedicated to validation
Throughout the implementation phase, customers have the possibility to observe the work progress and participate in the design of the finished product by having access to the validation site of the study. This site, accessible via the Internet, allows for the intermediate and final validation prior to signing the PV of beginning of production.
Our news
EOL New brochure available!
March 2016 : EOL the new brochure is available!
Medsharing in figures
(Figures 2015)
78,000 patients since 2003 for over 130 studies.
26 Registries / Observatories.
49 Studies.
61 Clinical Trials
31 Randomizations (without eCRF)
47 Drug Studies
28 DM Studies
66 Ongoing studies.
Figures 2015
New version of the application of randomization
Check out the new unlimited version of our Randomization for clinical trial application IPHONE / IPAD.
Quote request
+33 (0)1 48 75 39 14
by e-mail