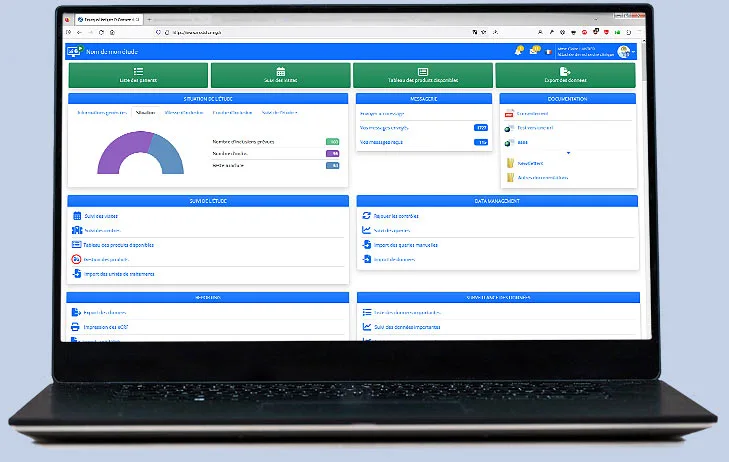
All modules, from design to randomization, monitoring, data management, and administration, are accessible online.
Advantages for your studies :
- Shared maintenance and hosting costs,
- 24/7 data availability,
- Confidentiality and security tailored to healthcare requirements,
- Fast and simple deployment,
- Agility to create/manage sites and investigators during the study,
- Agility to create/manage sites and investigators during the study,
- Full autonomy for your teams throughout the study.